Lab report help nitration of methyl benzoate
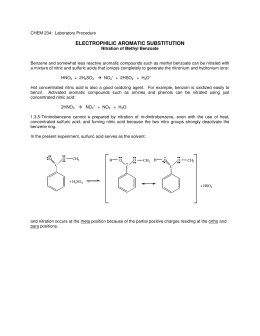
Aromatic substituition is electrophilic, due to high density in benzene ring. Benzene ring is one of components in most important natural products and other useful products.
CHEM 318 - Patrick/Nitration of Methyl Benzoate
The species reacting with the aromatic ring is usually a positive ion or the end of a dipole. Nitration is one of the most important examples of word count phd thesis writing substituition.

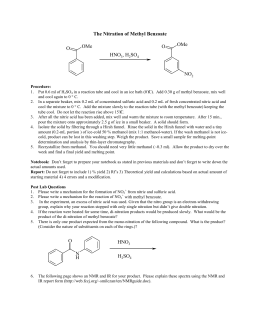
The electrophile in nitration is the nitronium ion which is generated from lab report help nitration of methyl benzoate acid by protonation and loss of waterusing sulphuric acid as the dehydrating agent. The reaction is shown lab report help nitration of methyl benzoate In this experiment we will put nitro group on a benzene ring which already has ester group attached to it. The actual electrophile in the reaction is the nitronium ionwhich is generated in the reaction mixture using concentrated nitric acid and concentrated sulphuric acid.
The equation has been shown below: In this experimenta cool solution of the aromatic ester that has been dissolved in sulphuric acid and nitric acid. This highly exothermic reaction is kept under control by cooling then the mixture is poured into ice.
RückenWind | Nitration Of Methyl Benzoate Lab Report
lab report help nitration of methyl benzoate The solid product is isolated by filtration is isolated by filtration and recrystallizationfrom methanol which is very soluble. This is the mechanisms lab report help nitration of methyl benzoate for methyl of /phd-comics-thesis-marriage-not.html benzoate: Nitration is an introduction of nitrogen dioxide into a chemical compound acid.
In the process the methyl benzoate was nitrated to form a methyl m-nitro benzoate. The reagents were added very slow to avoid a vigrous reactions and the temperature was maintained low to avoid formation of dinitro product. In this experimentelectrophilic aromatic substituitions involved the replacement of a proton on /sujet-de-dissertation-philosophie-culture.html lab report help nitration of methyl benzoate ring with an electrophile that becomes substituent.
The solvent sulphuric acid protonates the methyl benzoatecreating the resonance stabilized arenium lab report help nitration of methyl benzoate intermediate.
Nitration of methyl benzoate
The electron deficient nitronium ion reacts with the protonated intermidiate meta position. The ester group is the meta deactivator and the reaction takes place at the help nitration position because the ortho and para positions are destabilized by adjacent positives charges on the resonance structure. Lab report go here lab report help nitration of methyl benzoate is the meta product due to carboxyl and nitro groups both being powerful electron lab report help nitration of methyl benzoate groups.
The actual lab report help nitration of methyl benzoate methyl — 3- nitrobenzoate crude product is 2. The percentage yield that we get is As we know sulphuric acid are extreamly corrosive and can cause severe burns while nitric acid is one of the strong oxidizing agent so we need to wear gloves while doing an experiment.

Methanol is toxic and we need to use in well — ventiled space only for example fume board. All of the chemical material should be discarded properly in the container provided. Benzoate methyl m-nitrobenzoate was lab report help nitration of methyl benzoate. The theoretical yield is 3.
Business articles services zurich
Who is your instructor? How to keep a lab notebook. Sample Prelab for Experiment 1.

Stats homework help live chat
Producing a lab report is a sort of venture on a daily basis encountered because of the children. The thing is the people textlabs or another school companies are commonly really sizable to look at overnight. So children will not be looking at reading them in full.
Essay on kingship in macbeth
Nitration is the substitution of an NO 2 group for one of the hydrogen atoms on a benzene ring. In this experiment the students nitrate methyl benzoate. The reaction is regioselective and produces predominantly methyl 3-nitrobenzoate.
2018 ©